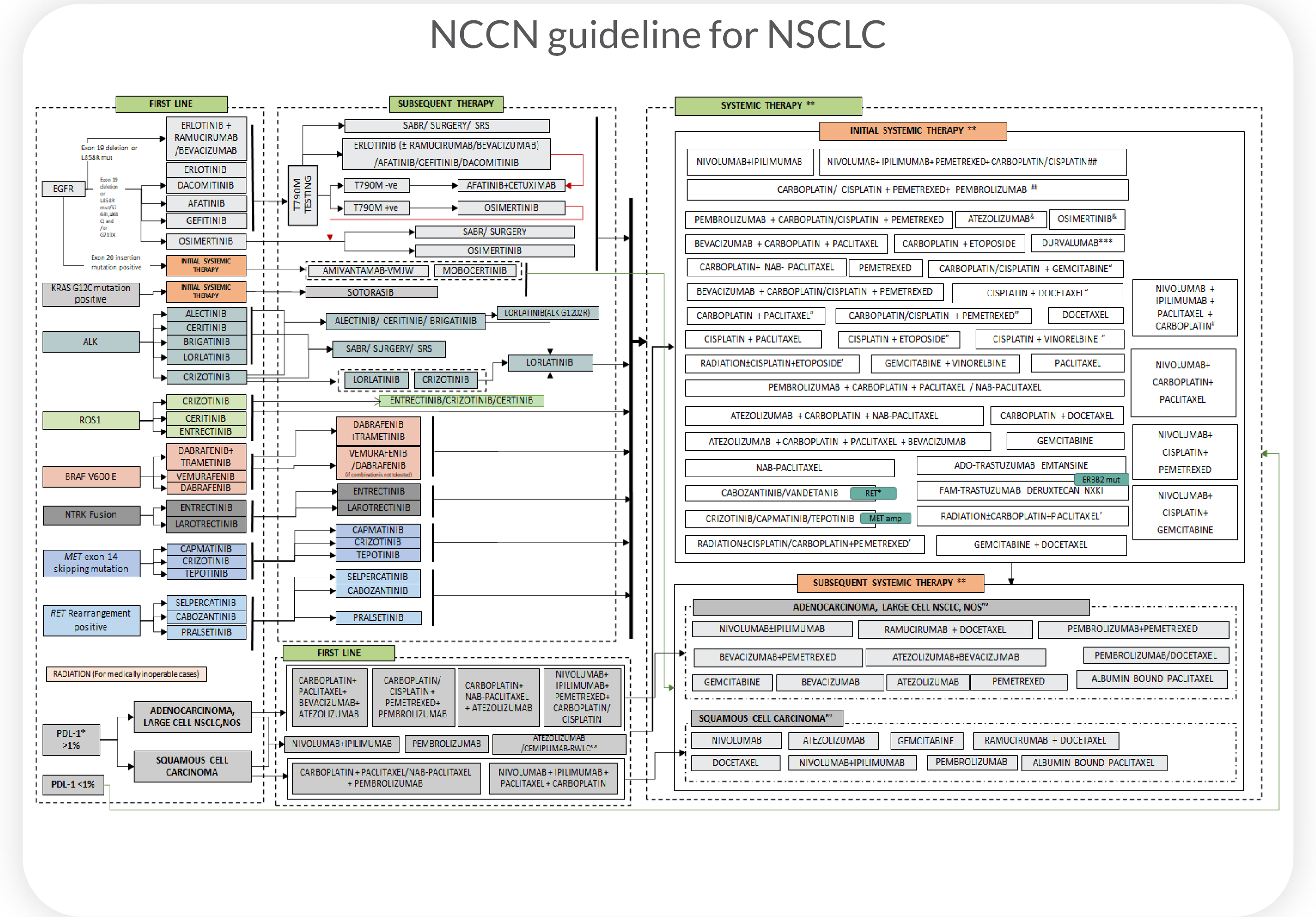
Current treatment guidelines typically
target one mutation with one drug, resulting in
low response rates across cancer types
Mutation
Enasidenib
Skipping Mutation
Capmatinib
Mutation
Pembrolizumab
Go beyond population-based treatment guidelines
to personalized therapy predictions
POPULATION-BASED
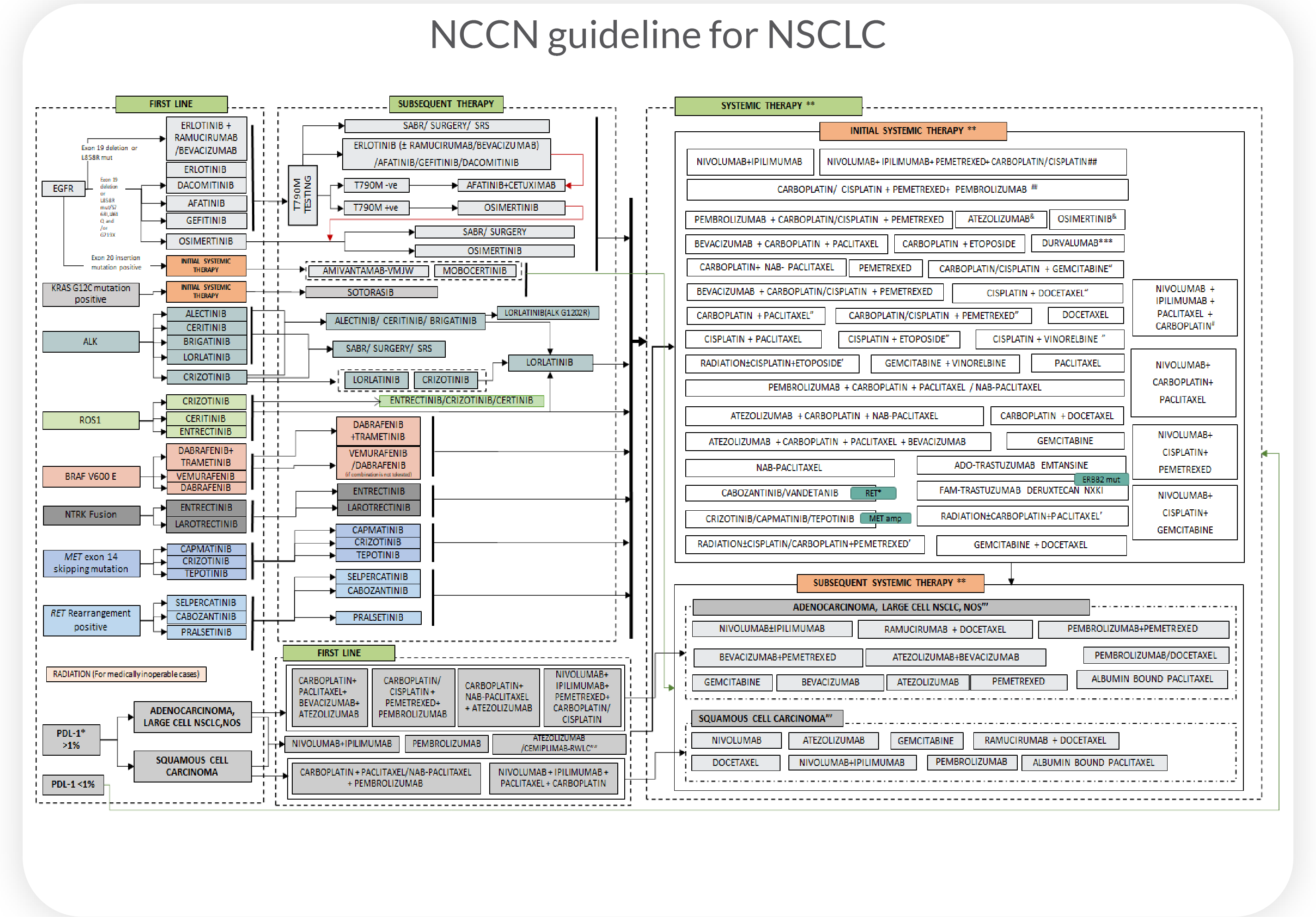
Population-based guidelines treat cancers of the same tissue type as genetically identical and overlook heterogeneity leading to low treatment response rates
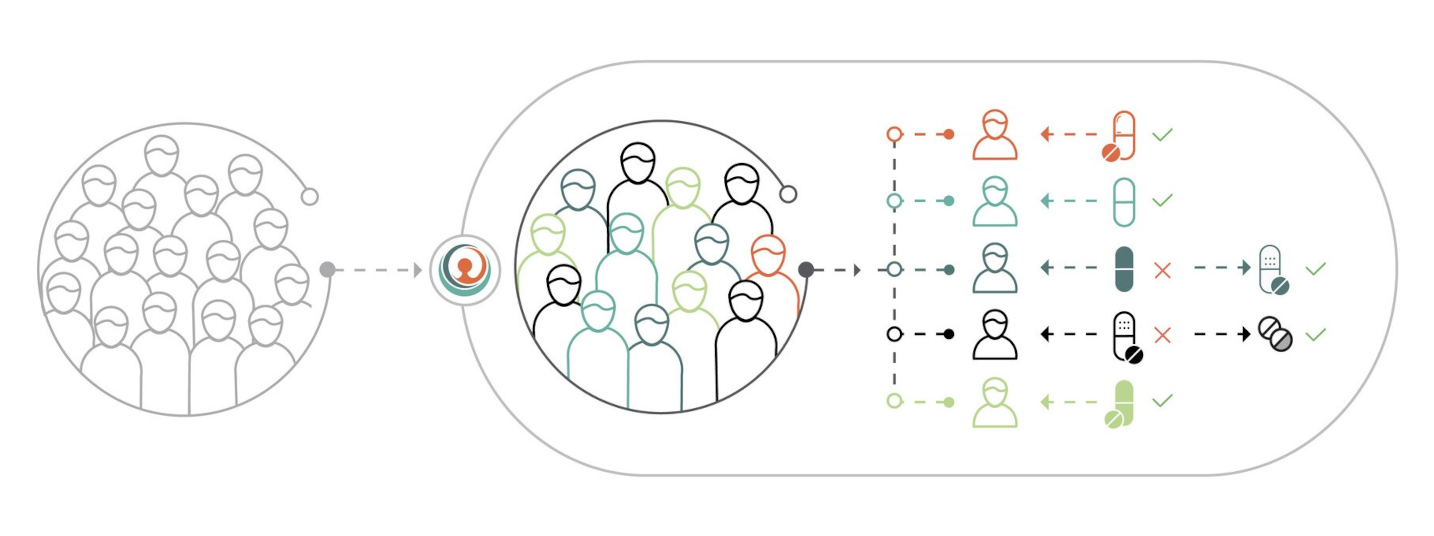
PERSONALIZED
Personalized therapy biosimulation predicts the impact of treatments on each patient's unique in silico disease model improving patient outcomes
Go beyond population-based treatment guidelines
to personalized therapy predictions
Personalized Therapy Biosimulation
Considers a patient’s
multi-gene mutations
and interactions
Delivers phenotype
predictions and identifies
patient-specific optimal therapies
Goes beyond NGS to
provide predictions
based on multiomic
data
Why biosimulate cancer
therapy responses?

Know response before treatment
Select personalized therapies
Avoid ineffective treatments
Improve patient outcomes
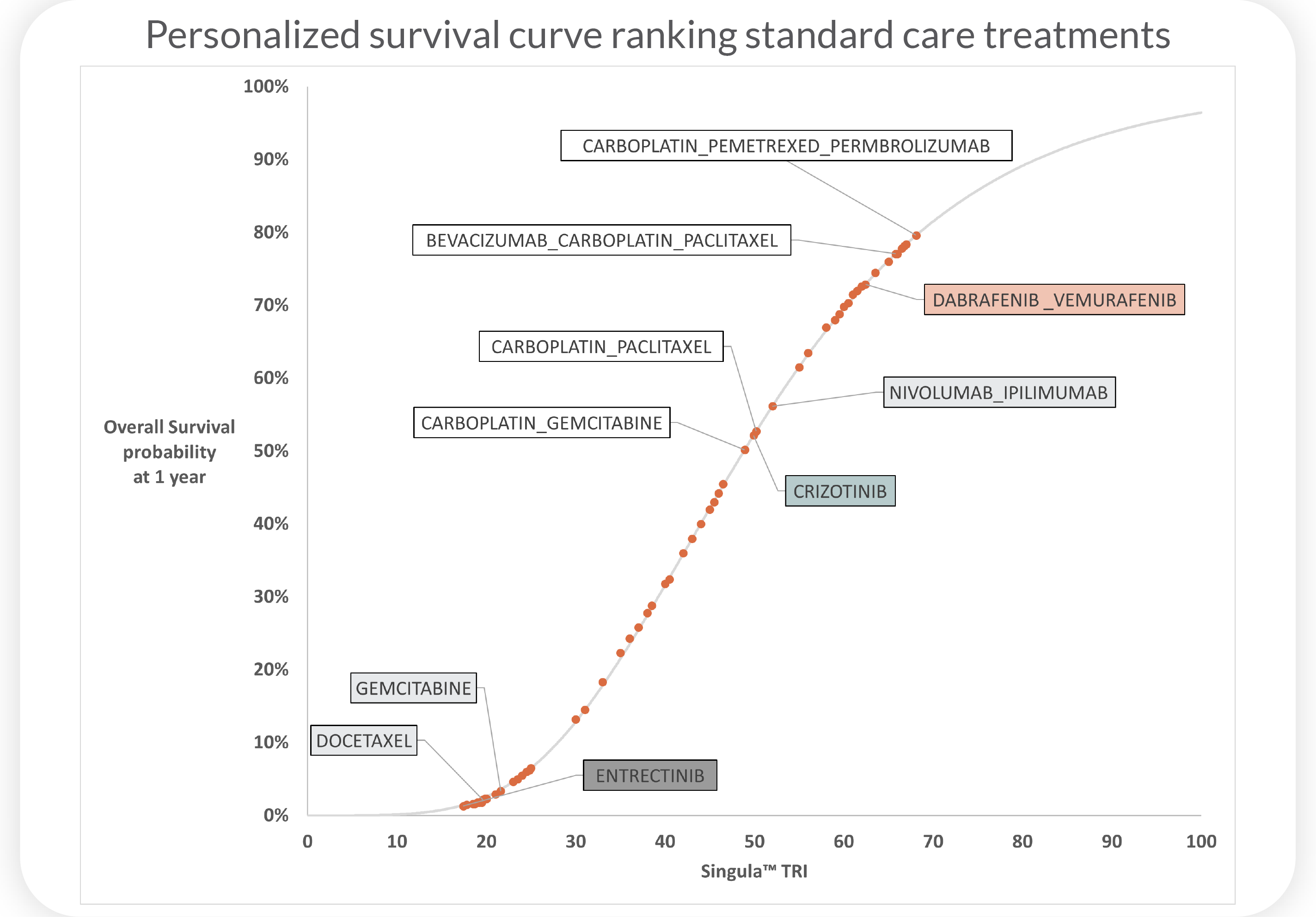
How does personalized therapy biosimulation work?

Biosimulate a patient’s multi-omic profile to predict and rank individual patient responses to millions
of drug combinations by identifying oncotecture master regulators, mechanisms of resistance and
conducting signaling pathway impact analysis.
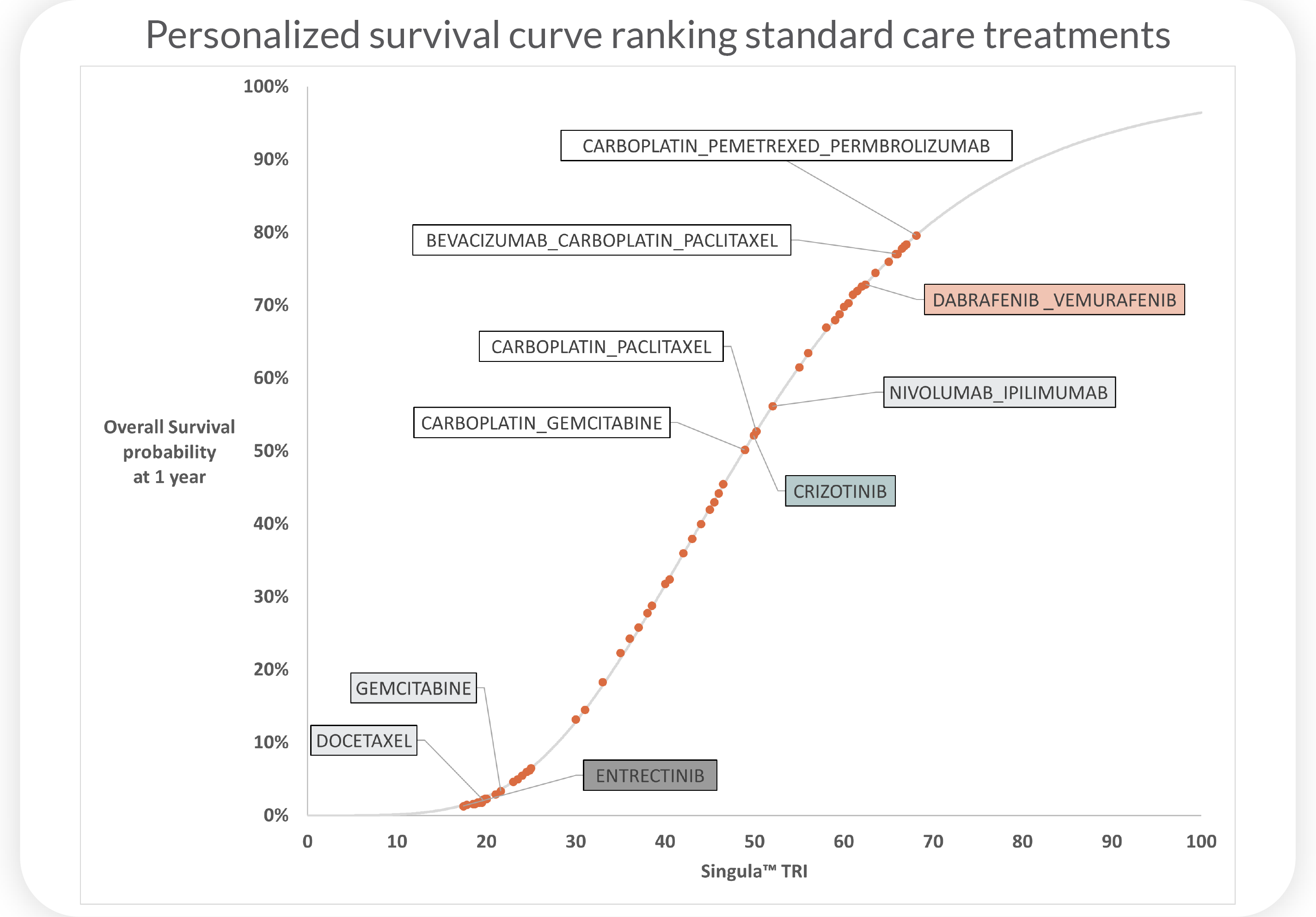
Therapy biosimulation reports predict each
patient's unique response to cancer treatments
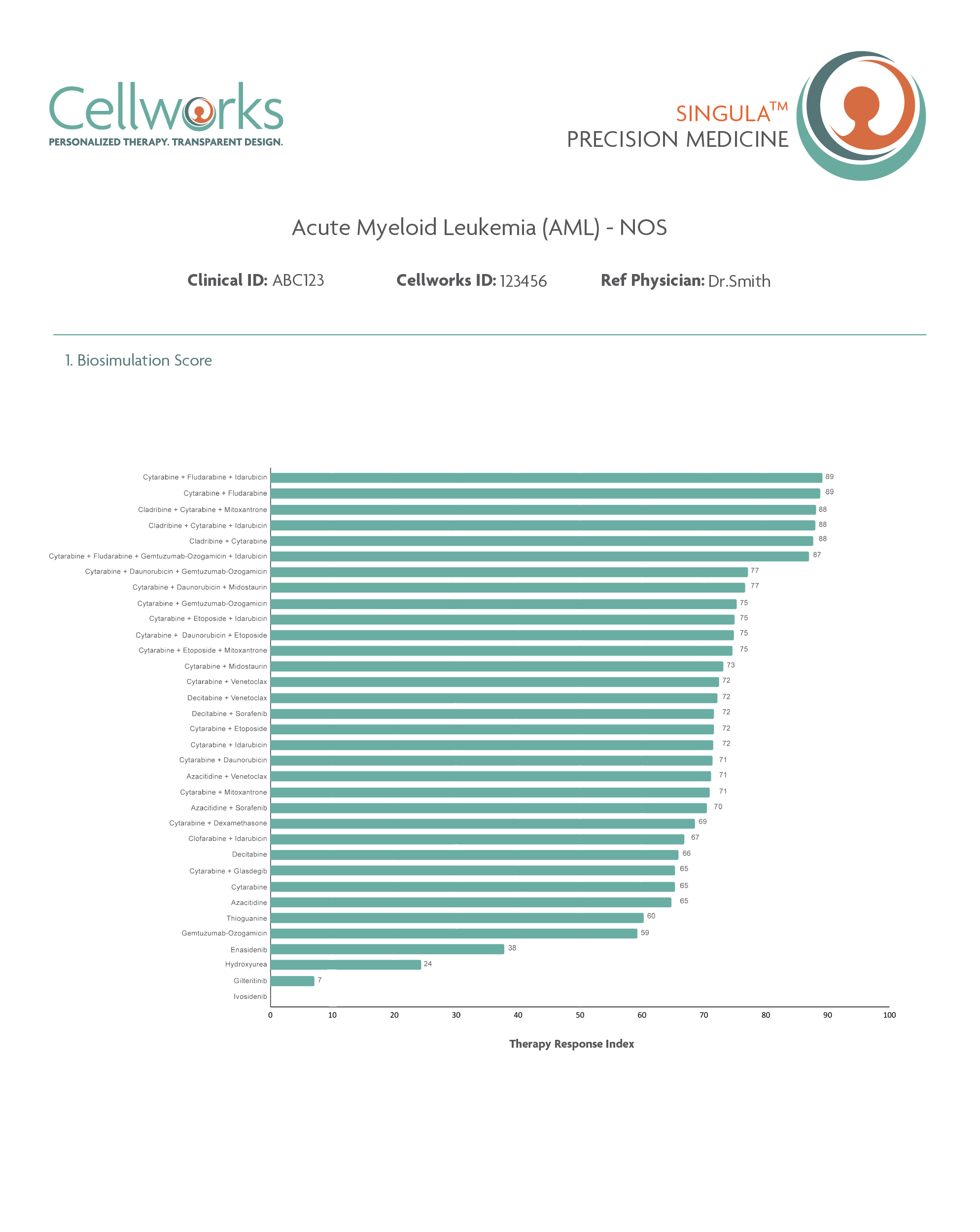
SINGULA™
Predicts personalized response to Standard Care therapies for front-line patients
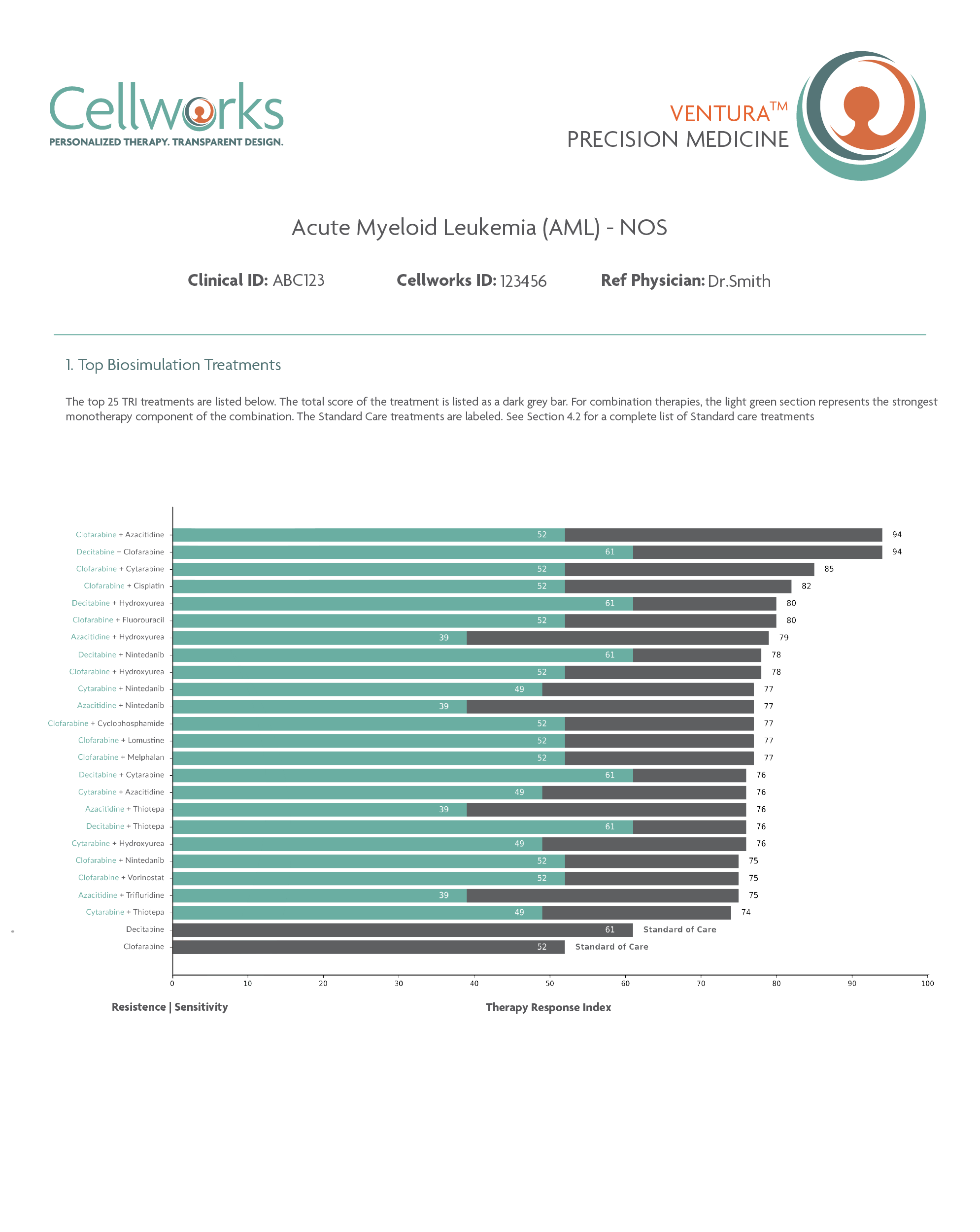
Download Sample ReportVENTURA™
Predicts and ranks personalized response to combinations of
FDA-approved drugs including off-label and non-oncology drugs for refractory patients
Cellworks biosimulation predictions proven
accurate across indications
Clinical Trial Process
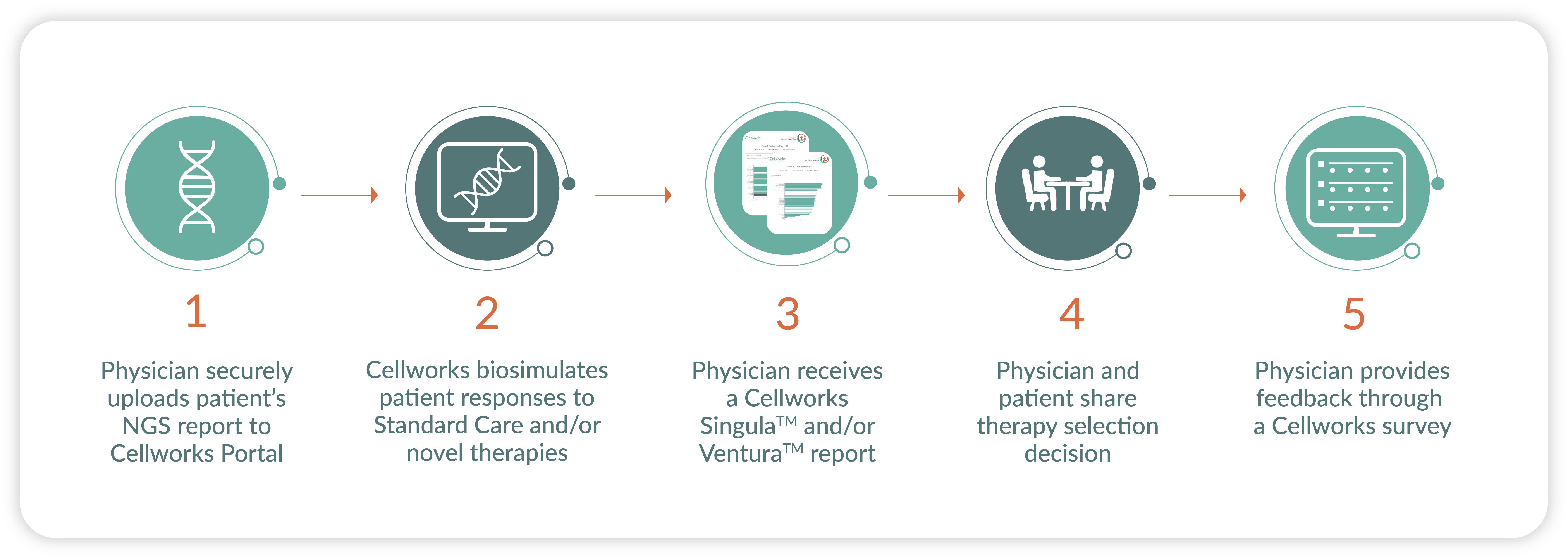
Principal Investigators
Who can participate in myCare-101 and myCare-102?
Physicians treating patients utilizing NGS reports for any of the listed
cancer indications can participate
- Bladder
- Bone
- Brain
- Breast
- Esophagus and Stomach
- Extragonadal Germinal Cell Tumors
- Genital Tract, Lower - Female
- Genital Tract, Lower - Male
- Head and Neck
- Kidney
- Large Intestine and Anus
- Leukemia
- Liver and Bile Duct
- Lung and Bronchus
- Lymphoma - Hodgkin
- Lymphoma - Non-Hodgkin
- Melanoma
- Mesothelioma
- Myelodysplastic Syndrome
- Myeloma
- Myeloproliferative Disease
- Ovary and Fallopian Tube
- Pancreas
- Prostate
- Skin
- Small Intestine
- Soft Tissue
- Testes
- Thymus
- Urothelial Carcinoma
- Uterus
- Cancer of Unknown Primary
- Bladder
- Bone
- Brain
- Breast
- Esophagus and Stomach
- Extragonadal Germinal Cell Tumors
- Genital Tract, Lower - Female
- Genital Tract, Lower - Male
- Head and Neck
- Kidney
- Large Intestine and Anus
- Leukemia
- Liver and Bile Duct
- Lung and Bronchus
- Lymphoma
- Lymphoma - Hodgkin
- Lymphoma - Non-Hodgkin
- Melanoma
- Mesothelioma
- Myelodysplastic Syndrome
- Myeloma
- Myeloproliferative Disease
- Ovary and Fallopian Tube
- Pancreas
- Prostate
- Skin
- Small Intestine
- Soft Tissue
- Testes
- Thymus
- Urothelial Carcinoma
- Uterus
- Cancer of Unknown Primary
Supported NGS Report Vendors
Sema4
Foundation Medicine
Caris
Tempus
MSK-IMPACT
Illumina